Through scientific testing and validation as well as IP protected processing, Kisolite is a standardized, consistent, safe and GLP-compliant product.
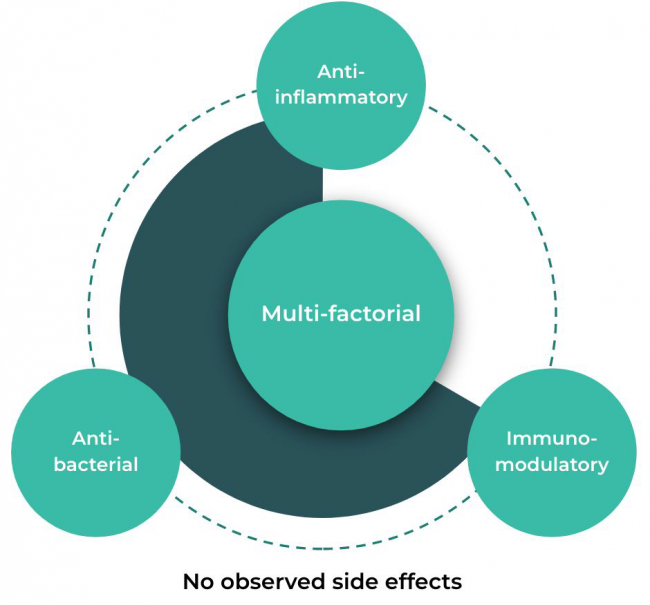
Kisolite is a naturally occurring bioactive mixture of minerals unknown to exist anywhere else in the world and is proven to have multi-factorial biological properties including anti-inflammatory, immunomodulatory and anti-bacterial effects.
Kisolite demonstrates significant efficacy for multiple pharmaceutical and consumer health applications, with no observed side effects.
Science
4D Labs
Internal R&D activities are conducted by Kisolite Corp’s Scientific team at 4D Labs, a leading materials science research institute based at Simon Fraser University in British Columbia, Canada.
Kisolite Corp is conducting scientific research focused on understanding the material’s bioactivity and composition. Studies to date have resulted in a standardized, consistent, safe, GLP-compliant product.
Althea DRF Lifesciences
Initiated in 2021, first pre-clinical developments have been conducted by Althea DRF Lifesciences at their FDA certified facilities under the direction of Kisolite Corp’s Scientific team.
Internally and in collaboration with Althea DRF Lifesciences, investigations of possible mechanisms were performed using cell-based models, which were then followed by evaluating efficacy in more advanced models that most closely mimic the actual human condition.
StratiCell
Initiated in 2022, additional pre-clinical studies are being performed at StratiCell, a leading company in pre-clinical skin R&D and testing, at their FDA certified facilities in Les Isnes, Belgium under the direction of Kisolite Corp’s Scientific team.
These studies aim to better understand the genetic basis of Kisolite's beneficial effects on skin health.
APR Applied Pharma Research s.a.
Initiated in 2023, APR, a Switzerland based pharmaceutical and research company provides Kisolite Corp. with high-value added contract services for development and testing of a pharma formulation for acne.
ETAP - Lab
Initiated in 2024, in vivo studies for inflammatory bowel disease (IBD) are being performed at ETAP-Lab, a French contract research organization.
IP Protected Processing Technology
Kisolite Corp’s Manufacturing Facility in Squamish, British Columbia, Canada
Kisolite is processed at the Company’s GMP compliant manufacturing facility under rigorous material handling, processing and testing conditions.
Using our established, IP-protected processing technology certified to meet industry standard requirements, we guarantee a safe and consistent ingredient for pharmaceutical and consumer healthcare markets.
Kisolite is 100% natural and does not contain any chemical or biologic additives.
Using Kisolite